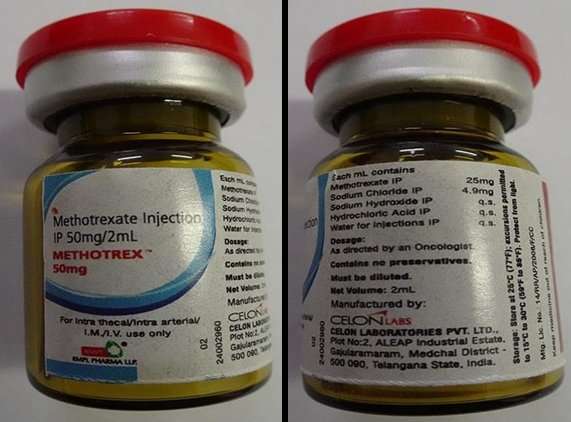
The National Agency for Food and Drugs Administration and Control (NAFDAC), has identified one batch of substandard (contaminated) Methotrextm (methotrexate) 50mg in the WHO Eastern Mediterranean region.
In a public alert message on its website, the agency implored healthcare providers to always exercise caution and vigilance within the supply chain to avoid the use of falsified or substandard medicinal products.
Methotrexate is a chemotherapy agent and immune system suppressant. It is used for the treatment of a wide variety of cancers as well as severe psoriasis, severe rheumatoid arthritis, and juvenile rheumatoid arthritis. It may be given by intrathecal, intramuscular, intravenous, or intra-arterial routes.
The agency highlighted details of the contaminated product are as follows: Name-Methotrextm 50mg; declared active ingredient- methotrexate 50mg/2mL; stated manufacturer- Celon Laboratories, Pvt Ltd – Telangana State, Indial; stated to be marketed by- RMPl Pharma llp – Mumbai; Batch number- MTI2101BAQ; Expiry date- 12/2022; Date of manufacture- 01/2021.
NAFDAC stated that the product’s batch number, production date, and expiration date have all been verified by the claimed manufacturer, Celon Laboratories Pvt Ltd., to match their internal records. They haven’t, however, gained access to samples of the questionable goods to do their own confirmatory tests.
Following complaints of adverse reactions in young kids using the prescription, health officials in Yemen and Lebanon tested the remaining, unopened vials of Methotrextm 50mg for microorganisms. It was reported that pseudomonas aeruginosa results were positive in both nations, indicating product contamination.
Pseudomonas aeruginosa bloodstream infection is a serious infection that may lead to death and any product that has any contamination and is administered directly in the body would present serious risks to patients.
According to the statement, “MTI2101BAQ, a 50 mg batch of Methotrextm, was only supposed to be distributed in India. The fact that the batch is still available in Yemen and Lebanon indicates that the product was obtained outside of the permitted supply chain, because this product was not intended for certain markets; the manufacturer cannot guarantee its safety”.
The drug according to NAFDAC is absent from their database. To prevent the importation, distribution, sale, administration, or use of fake or subpar pharmaceuticals, the agency urged importers, distributors, retailers, and healthcare practitioners to constantly exercise caution and attention along the supply chain.










