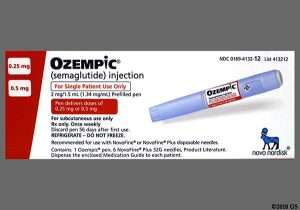
The National Agency for Food and Drugs Administration and Control (NAFDAC) has alerted the public on the circulation of one batch of Ozempic pen, confirmed to be counterfeit in Nigeria.
The recall is contained in a public alert with No: 014/2023 signed by NAFDAC Director General, Prof. Mojisola Adeyeye, in Abuja on Monday.
Ozempic (semaglutide) injection 0.5 mg, 1 mg, or 2 mg is an injectable prescription medicine. It is a once-weekly medicine for adults with type 2 diabetes used to improve blood sugar, along with diet and exercise. It also helps to reduce the risk of major cardiovascular events such as heart attack, stroke, or death in adults with type 2 diabetes and known heart disease.
In response to a consumer inquiry, Novo Nordisk, the product's marketing authorisation holder (MAH), verified that the MP5B060 batch of Ozempic (semaglutide) injection pen is counterfeit. Although no actual samples were sent back to the Nigerian Novo Nordisk office for examination, the picture of the allegedly falsified product was examined closely, and it was found that the pen depicted in the photo was different from the pen shown in the real product.
According to the MAH, there were a lot of cases from the Middle East and Russia that involved faked Ozempic. Azerbaijan, Egypt, Iraq, Jordan, Lebanon, Turkey, Uzbekistan, and Russia are among the countries that are hit.
Investigation conducted on the received falsified pens concludes that the pens are relabelled Apidra Solostar pen. The content of one of the pens was analysed and found to contain the fast-action insulin glulisin which is believed to be the case in all the falsified Ozempic pens identified. This implies the content of the falsified pens is entirely different from the genuine product.
According to Adeyeye, the main difference between the counterfeit product and the genuine product is that the genuine Novo Nordisk Ozempic pens do not extend or increase in length when setting the dose. The scale drum increments are fixed doses, such as 0.25 mg, 0.5 mg, and 1.0 mg for Ozempic pens.
She said a counterfeit pen can be identified based on scale going from 0 to 80 units and extending out from the pen when setting the dose.
The falsified products have been sold both within the legal and illegal supply chain.
Although the product is not in NAFDAC database, it is likely that it might have been distributed in the country through informal markets. NAFDAC implores importers, distributors, retailers and healthcare providers and patients to always exercise caution and vigilance within the supply chain to avoid the importation, distribution, sale and administration or use of falsified or substandard medicinal products. All medical products must be obtained from authorized/licensed suppliers. The products’ authenticity and physical condition should be carefully checked.













