The President of the Pharmaceutical Society of Nigeria (PSN) Pharm. Ahmed Yakasai has led a team of NEC members, chairmen of technical and interest groups, chairman, BOF and chairmen of some PSN Standing and Adhoc committees, to pay a courtesy visit to the NAFDAC DG, Prof. Mojisola Adeyeye in her office, in Lagos, on Wednesday.
The visit, which happened to be the first official visit, made by the PSN delegation to the NAFDAG DG, since her resumption in office, was well attended by PSN representatives and utilised for cogent discussion on how to move the agency as well as the Society forward.
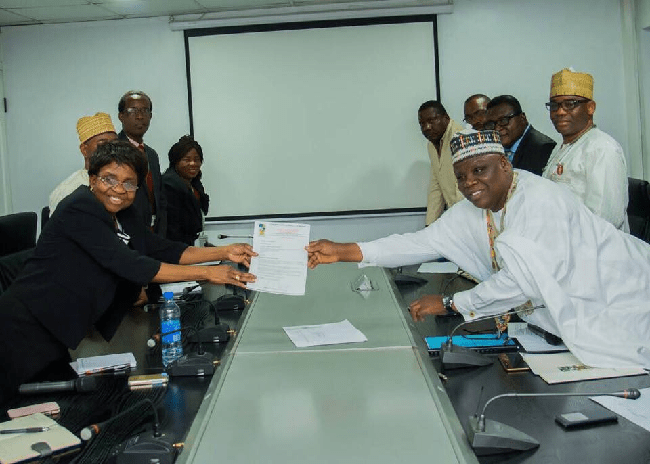
Having presented the needs of the Society to the DG, Pharm. Yakasai pledged the unalloyed support of PSN to NAFDAC, wishing her an uncommon success story as she steps into the office for the good of the Nigerian people, to achieve the safety margin in the regulation of Food and Drug sector of the economy.
Some of the issues raised at the meeting are:
Creation of an engaging process to obtain the perspectives of different stakeholders (Manufacturers, Importers, Distributors, Retailers etc.)
Strengthening the enforcement directorate of the agency to effectively and efficiently discharge its activities and at the same time heighten post registration surveillance to prevent distribution and sale of substandard and falsified products. Improved survey on quality selected drugs to generate reliable data is very necessary for an informed decision.
The need to strengthen the R&R Directorate, by adopting the robust regulatory information management system for efficient registration of products, to reduce the time it takes to register or renew regulated products.
Full implementation of National Drugs Distribution Guidelines (NDDG) – Federal Ministry of Health, NAFDAC, PCN, PSN, PMGMAN, NIROPHARM, and APIN – new take off date is 1st January, 2019
The need to promote high level of GMP standards among local manufacturing companies to facilitate the achievement of the goal of National Drug Policy and push for medicine security. NAFDAC can partner with some organisations to provide technical support to local companies.
NAFDAC to work towards becoming a member of Pharmaceutical Inspection Cooperation Scheme (PICS) which is for national regulatory agencies. NAFDAC needs to meet a minimum requirement to be accepted as a member. Once you become a member, any decision taken is accepted by other stringent regulatory authorities and become well respected globally. Only South Africa is a member in Africa. I believe USP can help in this direction.











