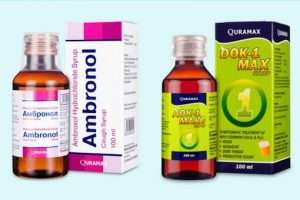
The National Agency for Food and Drugs Administration and Control (NAFDAC) has alerted Nigerians to two substandard products, Ambronol syrup and DOK-1 Max syrup, that were found in Uzbekistan, Europe and reported to the World Health Organisation (WHO).
The health agency which disclosed this information via its official website, advised manufacturers of liquid dosage forms to test for the presence of contaminants such as ethylene glycol and diethylene glycol before use in medicines.
Both products, according to the agency, included excessive levels of diethylene glycol and/or ethylene glycol as contaminants, based on the laboratory examination of the syrups, performed by national quality control labs of the Ministry of Health of the Republic of Uzbekistan.
Meanwhile, the WHO says it has not yet received any guarantee from the manufacturer, Marion Biotech Pvt. Ltd, on the items' quality or safety.
The statement further revealed that when taken by humans, diethylene glycol and ethylene glycol are fatally poisonous. Abdominal discomfort, nausea, vomiting, diarrhea, the inability to pass urine, headaches, changed mental status, and severe renal injury—which may result in death—can all be toxic consequences.
Substandard medical products are products that fail to meet quality standards or specifications and are therefore “out of specification”.
Due to their poor quality, NAFDAC noted that these products are dangerous, and using them, especially on children, might cause severe harm or even death.
The details of the substandard (contaminated) syrups are as follows: Product manufacturer: Marion Biotech Pvt. Ltd, (Uttar Pradesh, India); Product Names: Ambronol syrup and DOK-1 Max syrup.
“If you have these substandard products, please do not use them. If you, or someone you know, have used these products, or suffered any adverse reaction/event after use, you are advised to seek immediate medical advice from a qualified healthcare professional,” the statement read.
Even though the goods are not included in the NAFDAC database, importers, distributors, retailers, and customers are urged to exercise caution and attention throughout the supply chain to prevent the importation, distribution, sale, and consumption of the subpar (infected). syrups. All medical supplies must be purchased from approved and authorized vendors. Carefully examine the items' physical integrity and genuineness.













